Admins and Managers can access the STUDY > STUDY PROFILE: Section 3 eTMF Definitions. This section is where both the Site and Country folders and essential documents will be defined. This section will be pre-populated if either 1) an eTMF Reference Model has been selected for the study or 2) if the study was created based on another study with this section defined. If this section is not pre-populated and you want to define and track essential documents, then this section will need to be built out from scratch
NOTE: This is a convenience feature so that the same documents do not need to be created repeatedly, they will automatically be created and applied to all sites (the "reg pack") or applicable countries upon the creation of that site or country.
There are two parts in this section: The "SITES Folder and Document Definitions" and the "COUNTRY Folder and Document Definitions."
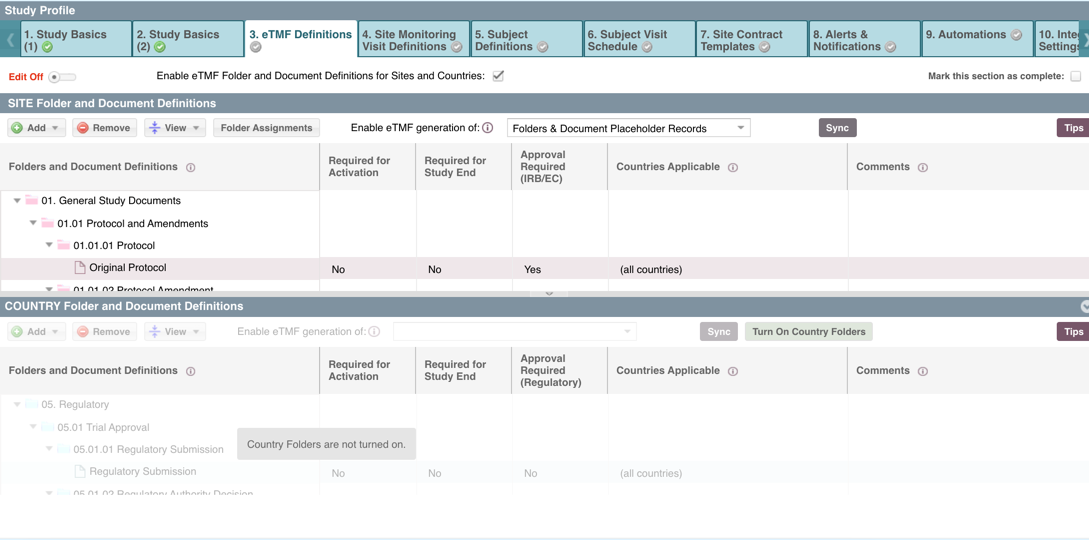
NOTE: In this example, the Countries section has not been turned on, that can be done using the "Turn on Country Folders" button but this cannot be reversed - whether you turn them on or off that can only be done once
Within this view Admins can:
- Define the site and country folder structures
- Define the site and country essential documents and:
- Specify those required for: study end, activation, and IRB/EC/RA approval
- Enable auto-generation of placeholders for those documents
- Assign where system-generated documents get saved (i.e. eVisit Reports)
- Activate/deactivate Country eTMF Definitions
Essential Documents differ from regular document records because they can have: additional study requirements tracked (i.e. IRB approval), have placeholder documents that are auto-generated by the system, and will be included in the PORTFOLIO > CTMS REPORTS Essential Document reports for tracking metrics.
The Document Records themselves are tracked within the DOCUMENTS view by the users responsible for managing the actual document record and file.
Placeholders are a type of document record and that have been auto-generated by the system within the DOCUMENTS view within a site folder and *only* contain the data added within this view. They should be modified by the user responsible for managing the site documents, to include document specific data (i.e. signature date, approval date) and if needed, have the document file attached to it. HINT: The "required" fields are not editable within the DOCUMENTS view. Below is an example of what placeholder documents look like within the DOCUMENTS view:
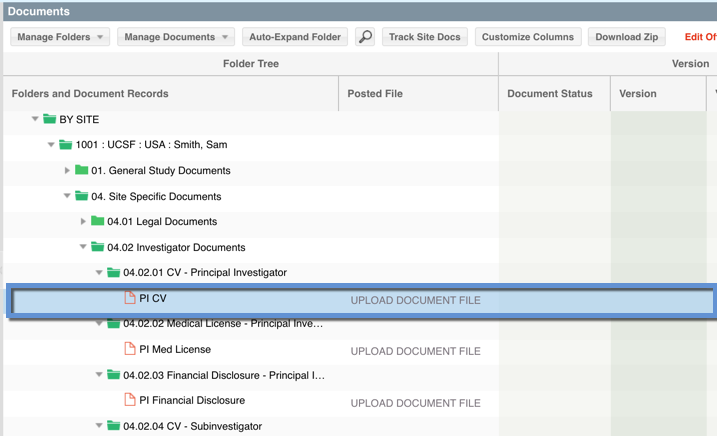
Site Essential Documents
Site Essential Documents are often call the "Reg Pack." Once the folders and essential documents have been defined, these folders and document placeholders will automatically populate within the PORTFOLIO > DOCUMENTS view immediately when sites are added within SITE > SITES & CONTACTS. One folder will automatically generate in the BY SITE folder for each site. Within that site, the defined sub-folders and if set up, essential documents placeholders will auto-populate.
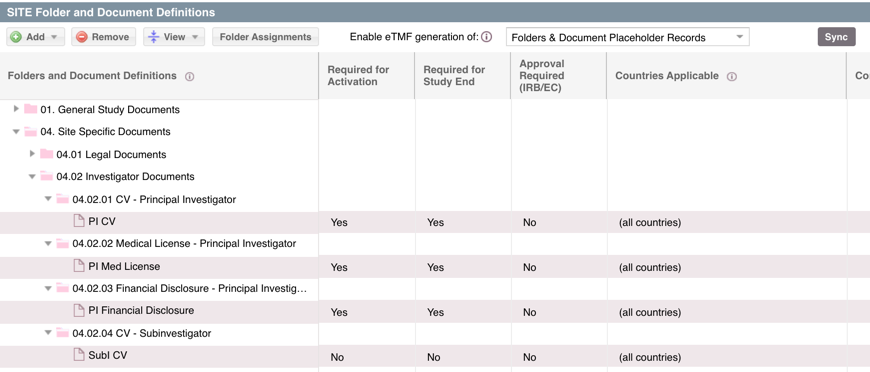
HINT: You can also apply an essential document definition to a document record that has been added to a site folder within the DOCUMENTS view. This will apply those defined requirements to the document record. See Can I mark a document as essential in the DOCUMENTS view? for more information on this process.
Country Essential Documents
Once the folders and essential documents have been defined, these folders and document placeholders will automatically populate within the PORTFOLIO > DOCUMENTS view immediately when countries are added within STUDY PROFILE: Section Two. One folder will automatically generate in the BY COUNTRY folder for each country. Within that country, the defined sub-folders and if setup, essential documents placeholders will also auto-populate.
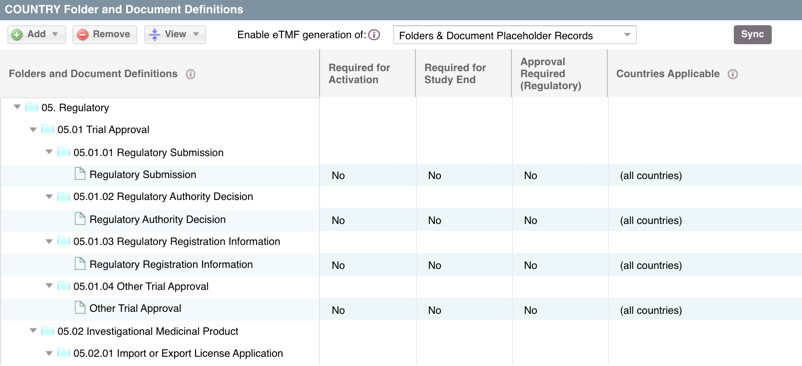
HINT: You can also apply an essential document definition to a document record that has been added to a site folder within the DOCUMENTS view. This will apply those defined requirements to the document record. See Can I mark a document as essential in the DOCUMENTS view? for more information on this process.
| Users with Access to this Functionality: | Managers and Admins |
| GLOSSARY |
|
| Essential Document Definition | The data attributed to a type of document record, within the Study Profile, that will be applied to document records |
| Essential Document Record | A type of document record that has certain study requirements applied to it (required for IRB approval) |
| Placeholder | A system generated document record based on the definitions in the Study Profile |
| Document Record | Within the Documents view, a row that captures the information about a document and allows for a file upload |
Comments
0 comments
Please sign in to leave a comment.