Within the eISF PORTAL, the "Documents for Upload" section is where site users can upload site documents for the study team to retrieve. The study team should create a list of the documents that they need from the site (i.e. a completed 1572, the PI CV, local lab credentials etc.) and those documents should automatically appear in the grid below with one row per document.
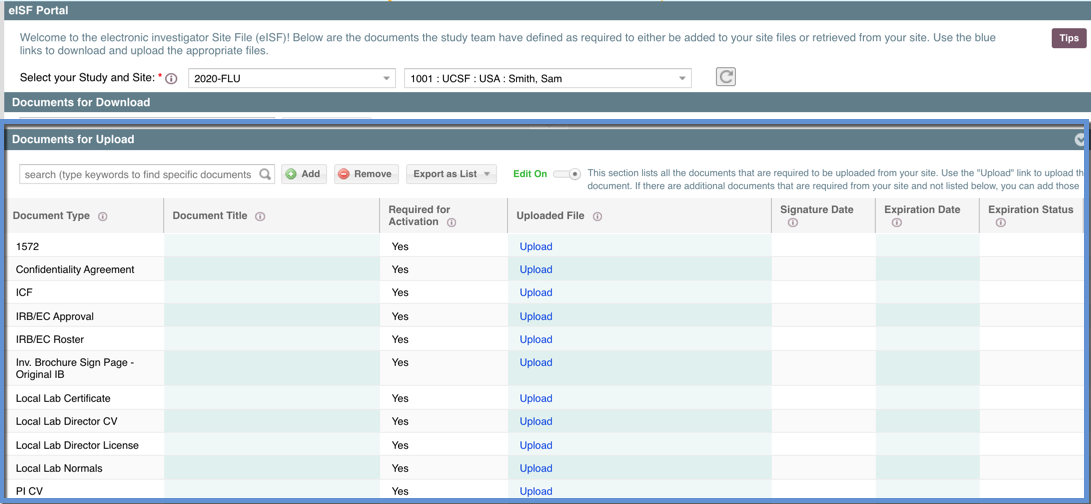
Within this section you can search for specific documents using keywords, sort columns by clicking on the column header, add "ad hoc" documents (additional documents the study team didn't define), remove those ad hoc documents, remove file attachments (with some limitations), input some document specific information, and export this list of documents as an external document.
Within each row are fields that pertain to that document (the green indicates which fields are editable):
- Document Type = This is general designation of what kind of document this is. This is for the study team for their study files (Trial Master File) to easily designate what kind of document this is (i.e. Sub-I CV, 1572, ICF) [Editable only for documents not defined by the study team]
- Document Title = The is the specific description of what document this is (i.e. "Sub-I CV Jones, Alex 18May2020" ; "ICF Approved 14Jan2021") [Editable only for documents not defined by the study team]
- Required for Activation = This field will say either "Yes" or "No." This is defined by the study team to determine whether a document has been designated as is required to be collected by the study team before the site can be activated for study participation. HINT: This may be used as the designation for a document being included in the startup or reg pack. [Not Editable]
- Uploaded File = This will either show an "Upload" link that can be clicked to launch a form where you can upload the corresponding file to that document. Or if that has already been done, it will show the filename of the attached document. [Not Editable]
- Signature Date = A field you can edit to include the date the document was signed [Editable]
- Expiration Date = A field you can edit to include the date the document expires [Editable]
- Expiration Status = A field you can edit to include the expiration status of the document [Editable]
- Comments = Any additional notes the study team included for your reference HINT: This may be used as the designation for a document being included in the startup or reg pack. [Not Editable]
- Posted Date = The date the study team included this document in the eISF PORTAL [Not Editable]
- Uploaded By = The name of the person who added the document file (in the "Uploaded File" column) [Not Editable]
- Date Uploaded = The date the document file was added (in the "Uploaded File" column) [Not Editable]
How to Upload Documents to the eISF
There are two ways to upload a document to the eISF Portal:
- By using the "Upload" link within an existing document record
- By using the "Add" button to add documents that are not pre-defined
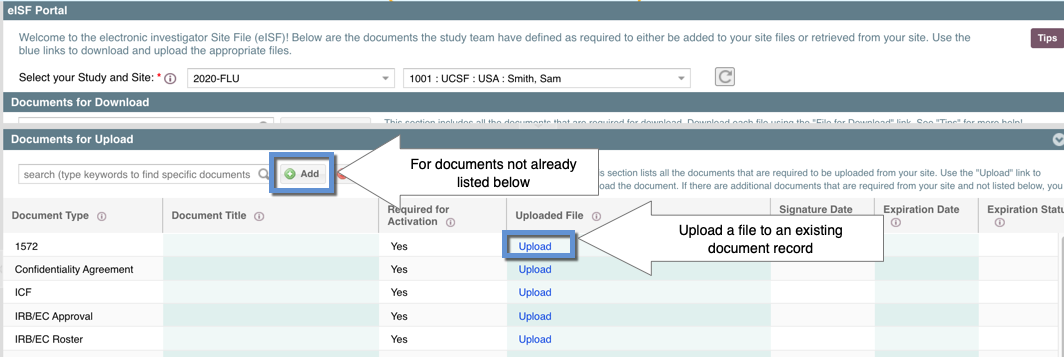
With both methods the "eISF Document Upload" form should appear.
Step One: Input the Document Title and (if applicable) the Document Type
- The Document Title should be descriptive enough the study team can easily identify it (i.e. "PI CV, Ali, Jermaine 15Jun2019")
- The Document Type can only be selected if it is an "ad hoc" document (created using the "Add" button for a whole new document, not an attachment to an existing document record). Select the one that applies to your document to make internal filing for the study team easier
Step Two: Opt Whether to Electronically Sign the File
If you do want to electronically sign a file you will need to input your email and SimpleTrials password
Step Three: Attach a File
There are two options to upload a file attachment
- Drag and Drop -
- Choose File - click this button to launch your local drives and select the file
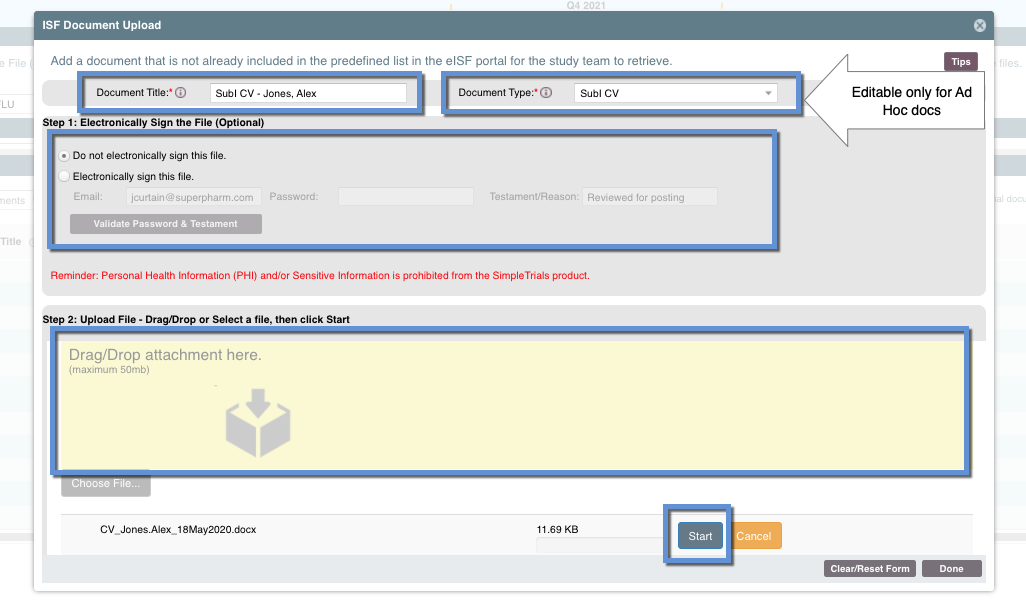
Step Four: Upload the File Attachment
Once the file has been attached, click "Start" to upload the file. Once that is complete, select Done.
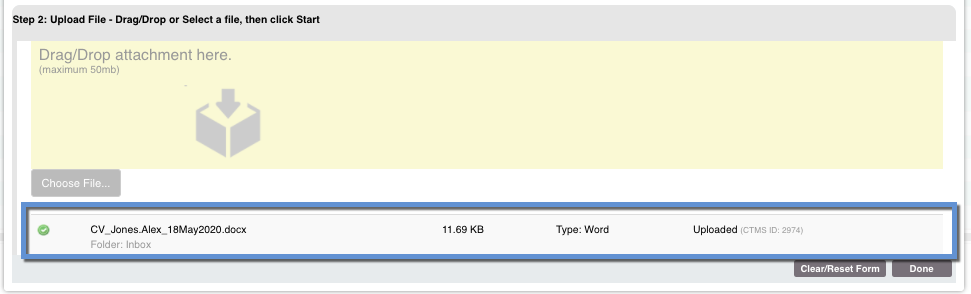
Once the file has been attached to the document, you should see it in the Document for Upload list with the file attached:
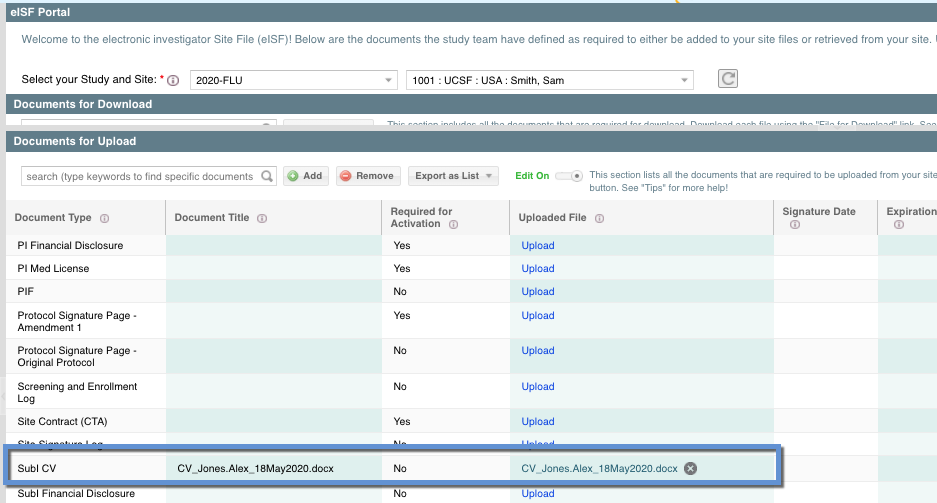
From there you can add the Signature Date, Expiration Date, and Expiration Status. You can also remove this attachment and document record if needed.
Note: If you select the "Add" button to add an ad hoc document and within the "eISF Document Upload" form you select a "Document Type" that is in the "Document for Upload "list that does not already have a file attached, the attached file will automatically be attached to that document record.
Additional Info:
What happens from there is these files are automatically sent to the study's electronic Trial Master File (eTMF) in SimpleTrials. Once the study team reviews the uploaded document and sends it to their eTMF site folder, that attachment can no longer be removed from the eISF PORTAL. If you have additional questions on the documents provided or requested in this view, please contact your study team contact.
| Users Access Requirements | Admin, Manager, Associate, Document Manager, Site Manager, eISF Collaborator |
| Portfolio View Access Requirements | All Data, Studies + General Records, Studies Only, Sites Only |
| Subscription Requirements | Trial, Premium, Premium Plus, Enterprise |
| Glossary | |
| eISF Portal | An electronic document exchange where the site staff can download study documents provided by the study team, and upload site documents for the study team to retrieve. It is NOT an electronic ISF or reg binder where site staff manage their site files. |
| eISF Collaborator | A User Role designed specifically for site staff who only need access to the eISF PORTAL to exchange documents with the study team |
Comments
0 comments
Please sign in to leave a comment.