If your study team is using the eISF PORTAL to define the list of the documents required to be collected from your site, they may also be further defining the list of documents to indicate those that are required to be collected from your site before it can be activated for participation in the trial. This collection of documents are often called the "Reg Pack," "Reg Docs," or "Startup Packet." They usually include such documents as the 1572, PI CV/ML, completed Financial Disclosure Forms, local IRB Approvals, site specific ICF, local lab credentials, etc.
If your study team has indicated a list of startup documents, they should inform you how to find them. The two most common methods are:
Option One: Required for Activation Column
The study team may be using the "Required for Activation" column. Where a document is indicated as "Yes" - that is a document that is required for site activation and part of the startup/reg pack. Click on this column header and the column will automatically sort by Yes/No.
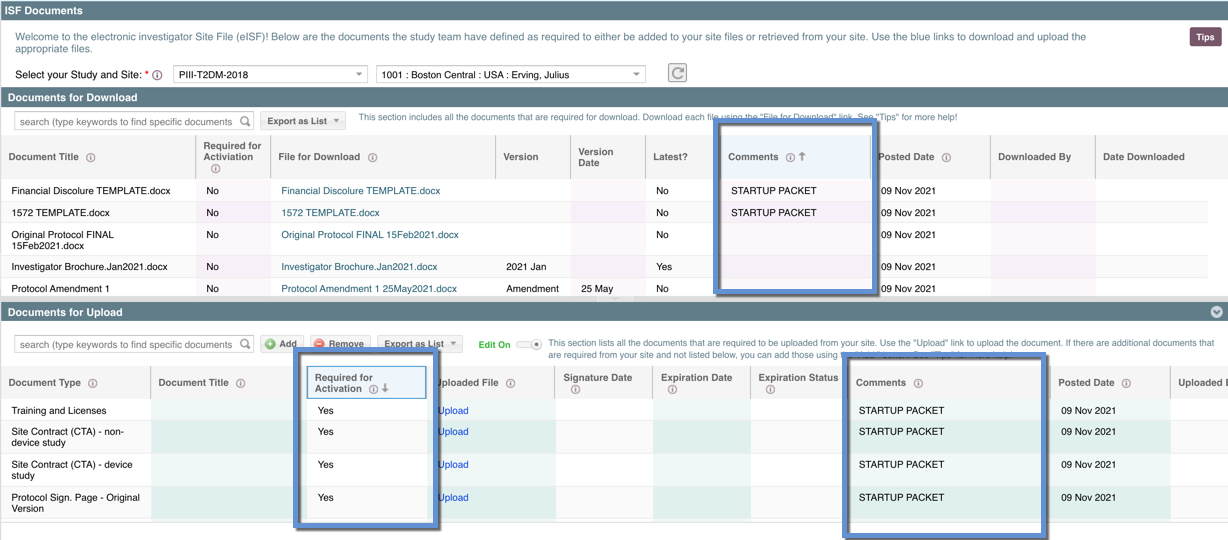
Option Two: Comments Column
The study team may be using the "Comments" column to indicate a document that is required for collection. Click on this column header and the column will automatically sort by comments, alphabetically.
This may be used in both in Documents for Upload and Documents for Download to indicate documents the site needs to download (i.e. the template 1572, template FDF, template ICF etc.). In the example above, the study team added "STARTUP PACKET" to all the documents that are part of required to be collected as part of the startup packet.
Option Three:
They study team may use BOTH of these methods. Since there is no "Required for Activation" column in the "Documents for Download" (as those are documents provided by the study team TO the site) they still may want to indicate a template that is part of the reg pack for the site to download, complete, and return to the study team.
| Users Access Requirements | Admin, Manager, Associate, Site Manager, Document Manager, Viewer, Site Viewer, Document Viewer, eISF Collaborator |
| Portfolio View Access Requirements | All Data, Studies + General Records, Studies Only, Sites Only |
| Subscription Requirements | Trial, Premium, Premium Plus, Enterprise |
| Glossary | |
| eISF Portal | An electronic document exchange where the site staff can download study documents provided by the study team, and upload site documents for the study team to retrieve. It is NOT an electronic ISF or reg binder where site staff manage their site files. |
| Startup Packet | AKA: Reg Pack, Reg Docs - These are the documents required to be collected by the site and reviewed be the study team before that site can be participate in the clinical trial |
| eISF Collaborator | A User Role designed specifically for site staff who only need access to the eISF PORTAL to exchange documents with the study team |
Other Relevant Articles:
How to Upload Documents to the eISF Portal
How to Download Documents from the eISF Portal
How to find specific documents in the eISF Portal
How to Create a New Site Document Record in the eISF Portal
Comments
0 comments
Please sign in to leave a comment.