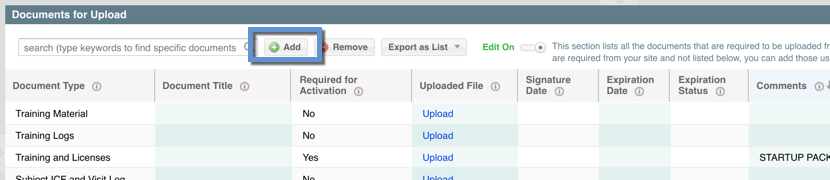
Within the eISF PORTAL is a list of documents that the study team has defined as needed to be collected from the site called "Documents for Upload." For each of those documents you can use the "Upload" link to attach a document file to that document record.
There may be instances where you need to upload a document that is not already included in this list. For instance, if you have multiple sub-Is or site specific documents - you may need to create documents that have not been pre-defined by the study team. To do this:
- Click "Add." This will open the "eISF Document Upload" form. This is the form where you will create a new document row within the Documents for Upload view and where you will upload the file attachment.
- Type the "Document Title" being sure to use identifiers so the study team can easily discern exactly which document this is (i.e. "Sub-I CV, Jones, Signed 15Nov2019")
- Select the "Document Type" being sure to select an appropriate existing type from the dropdown. This is used to help the study team manage and organize the documents retrieved from sites, into the correct folders in their electronic Trial Master File (eTMF). Only use "Other" if there is no Document Type that makes sense for your document
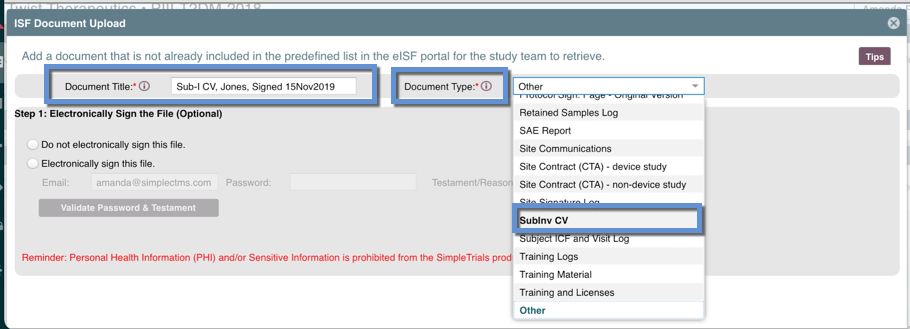
- Complete the steps to upload your document file and click "done"
- A new row should appear in the "Documents for Upload" view. From here you can add additional fields if necessary.
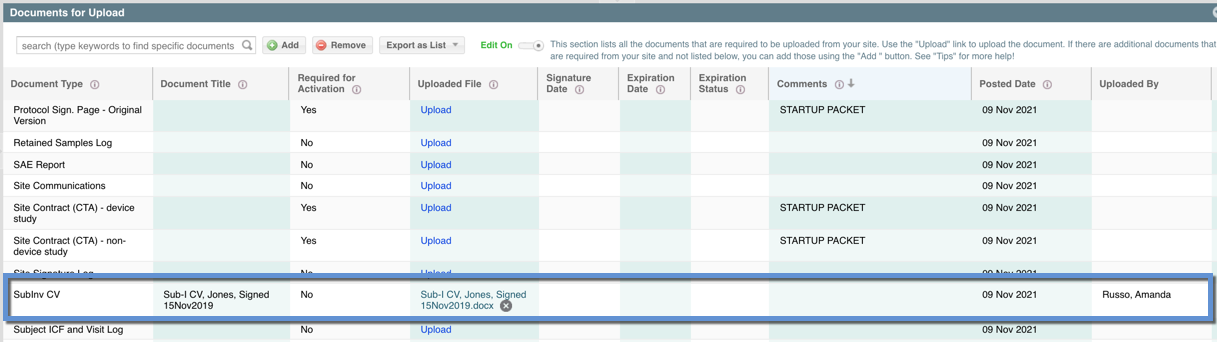 NOTE: If you use the "Add" button to add a new document and there is already an existing row for that document type without an attached document- your attachment will automatically be appended to the existing document row for that document type. This a convenience feature to prevent duplicates and allow the study team to track required documents.
NOTE: If you use the "Add" button to add a new document and there is already an existing row for that document type without an attached document- your attachment will automatically be appended to the existing document row for that document type. This a convenience feature to prevent duplicates and allow the study team to track required documents.
| Users Access Requirements | Admin, Manager, Associate, Site User - ST Manager, Document Manager, Viewer, Site User - ST Viewer, Document Viewer, eISF Collaborator |
| Portfolio View Access Requirements | All Data, Studies + General Records, Studies Only, Sites Only |
| Subscription Requirements | Trial, Premium, Premium Plus, Enterprise |
| Glossary | |
| eISF Portal | An electronic document exchange where the site staff can download study documents provided by the study team, and upload site documents for the study team to retrieve. It is NOT an electronic ISF or reg binder where site staff manage their site files. |
| Ad Hoc Document | A document record that is added by a site user and does not correlate to a document record defined by the study team |
| eISF Collaborator | A User Role designed specifically for site staff who only need access to the eISF PORTAL to exchange documents with the study team |
Other Relevant Articles:
How to Upload Documents to the eISF Portal
How to Download Documents from the eISF Portal
How to find specific documents in the eISF Portal
Comments
0 comments
Please sign in to leave a comment.