The initial set-up of your study involves completing the STUDY>STUDY PROFILE. This area is comprised of multiple sections and determines the framework for your study in numerous other areas of the system. The tab Section 2: Study Basics (2) captures details of the Study Protocols, Participating Countries, and Investigative Products.
To make any changes in this view, first ensure the Edit toggle is switched to "Edit On"
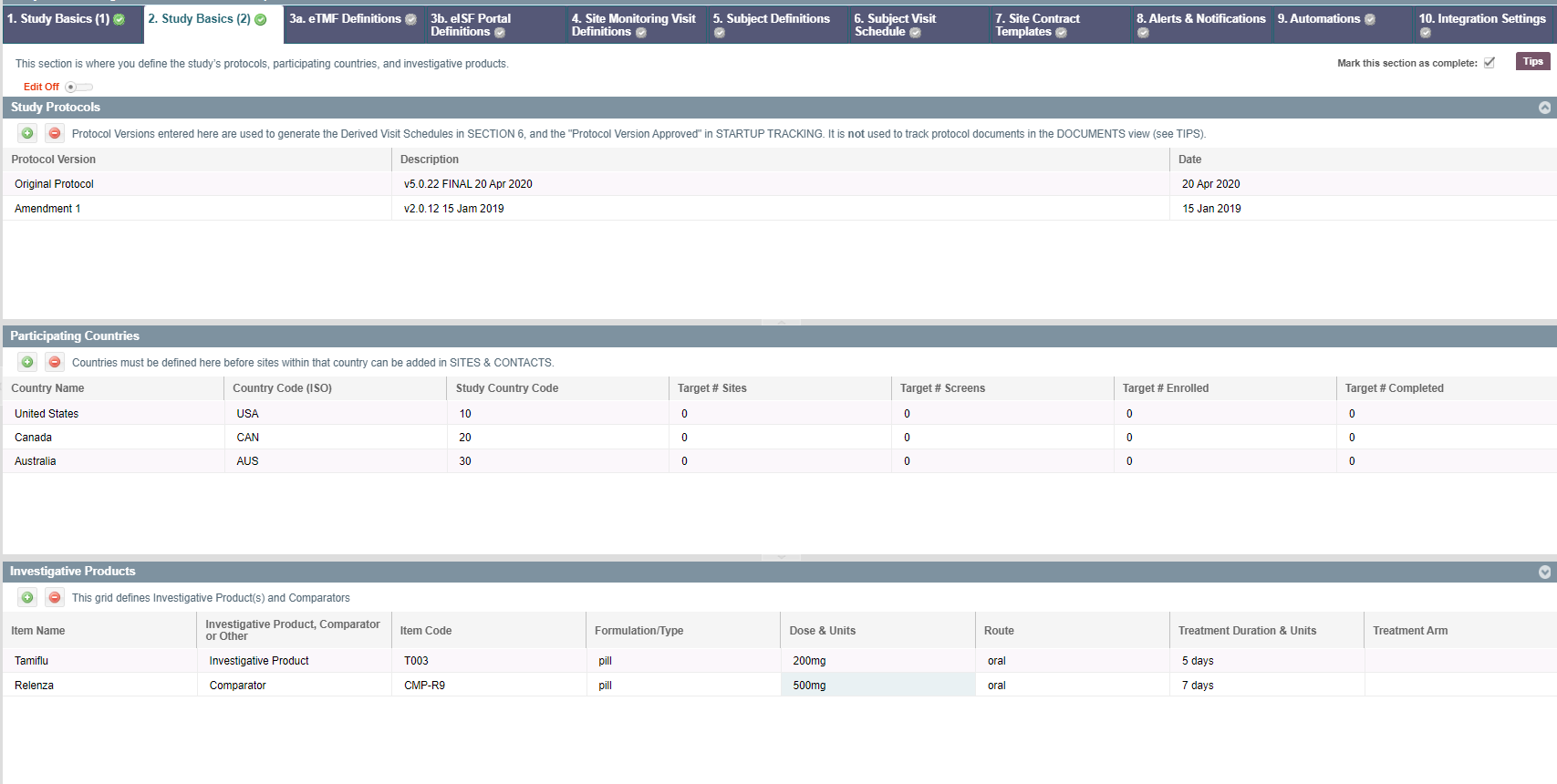
Study Protocols
The top grid in this view shows the Study Protocols. It is automatically populated with the "Original Protocol" at the creation of the study. The protocols created here will automatically be populated in Study Profile > Section 6 Subject Visit Schedules.
- Add or Remove - New protocols are automatically added to the bottom of the list and are named sequentially (Amendment 1, Amendment 2, etc). They are also removed starting from the last Amendment.
- Protocol Version - You can rename the Protocols here. This name will show up in Section 6 Subject Visit Schedules when you go to create visit schedules for the protocols.
- Description - Free text field that can be used to describe the protocol
- Date - Date field
NOTE: This is not where the tracking of the Protocol document is done. To track the Protocol document in the TMF use the DOCUMENTS/eTMF view.
NOTE: If the new Protocol Amendment includes changes to the study's milestones or tasks, be sure to make those changes in the MILESTONES & TASKS view.
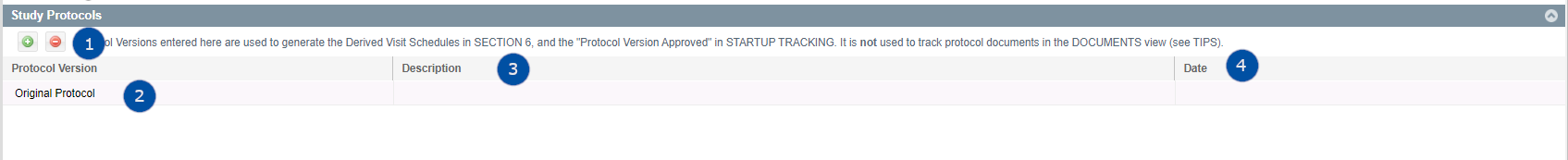
Participating Countries
This table is used to identify the countries where the study will take place. IMPORTANT NOTE: You must have countries defined here before you can add sites in SITES > SITES & CONTACTS or create contract templates in STUDY PROFILE > SECTION 7
- Add - This will open a picklist where you can select a country and that country's currency you would like to add.
- Country Name - The full name of the country added to the Study
- Country Code (ISO) - Auto-populated upon addition of country to this view
- Study Country Code (optional) - Text Entry to define an internal code to the country
- Targets (Optional) - Users may edit grid to add/remove countries or adjust target # of sites, screening, enrollment and completion values by country.
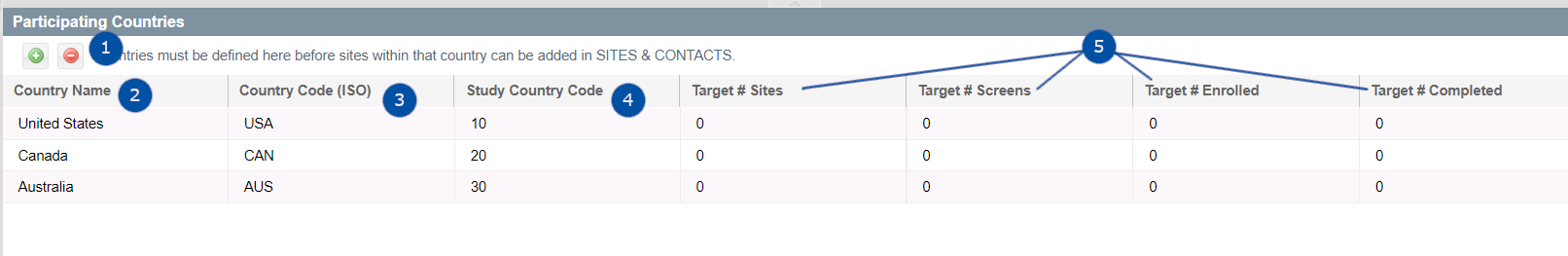
Investigative Products
This table is used to document the Investigative Product (IP) and Comparator products utilized in a given study as well as the dosing regimen, route and treatment duration.
- Add - Add new Item's to the list.
- Editable cells which can be used to document information about each IP or Comparator product.
| Users Access Requirements | Admin, Manager |
Comments
0 comments
Article is closed for comments.